Kaolinite, illite, montmorilonite, How the clay minerals kaolinite, illite and montmorillonite are formed by schematic diagram.
Kaolinite:-
- Kaolinite is the most common minerals of the kaolinite group of clay minerals.
- Its basic structural unit consists of gibbsite sheet with aluminium atoms at the center.
- It is joined to silica sheet through the unbalanced oxygen atoms at the apexes of silica sheet.
- The total thickness of the basic structural unit is about 7 angstrom.
- The basic structural unit of kaolinite minerals is symbolised as shown in figure.
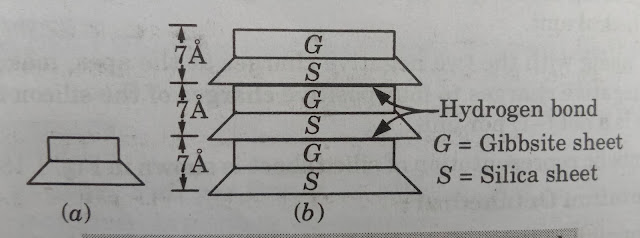
Kaolinite minerals - The basic structural units of kaolinite minerals are joined together by hydrogen bond. It develops between the oxygen atoms of silica sheet and the hydroxyls of gibbsite sheet.
- Since the hydrogen bond is fairly strong, it is extremely difficult to separate the layers, and hence, kaolinite minerals is relatively stable.
- Moreover, water cannot penetrate through the layers of the structural units of kaolinite minerals.
- Kaolinite shows relatively little swell on wetting.
- China clay is almost pure Kaolinite.
Montmorillonite
- Montmorillonite is the most common minerals of the montmorillonite group of clay minerals.
- Its basic structural unit consists of gibbsite sheet sandwiched between two silica sheets.
- The gibbsite sheet may include atoms of aluminium, iron or magnesium or a combination of these.
- The total thickness of basic structural unit of montmorillonite is about 10 angstrom.
- The basic structural units of montmorillonite minerals are joined together by a link between oxygen ions of the two silica sheets.
- The link is due to natural attraction for the cations in the intervening space and due to vander waal forces.
- Water may enter between the silica sheets causing the minerals to swell.
- For this reason, montmorillonite tends to wetting.
 |
| Montmorillonite minerals |
Illite:-
- Illite minerals is most common minerals of the illite group of clay minerals.
- Its basic structural unit is similar to that of montmorillonite minerals except that there is always substantial 20 percent plus minus substitution of silica atoms by aluminium atoms in silica sheet.
- Also, the link between the different basic structural units is pro non exchangeable potassium ions.
- The basic structural unit of light is symbolically represented as shown in figure.

Illite minerals - The swelling of illite is more than that of kaolinite, but less than that of montmorillonite.
- Thus, the properties of illite are somewhat intermediate between those of kaolinite and montmorillonite.


Comments
Post a Comment